3.5 Lipids
Created by: CK-12/Adapted by Christine Miller
Yum!

It glistens with fat, from the cheese to the fries. Both cheese and fries are typically high-fat foods, so this meal is definitely not recommended if you are following a low-fat diet. We need some fats in our diet for good health, but too much of a good thing can be harmful to our health, no matter how delicious it tastes. What are fats? And why do we have such a love-hate relationship with them? Read on to find out.
Lipids and Fatty Acids
Fats are actually a type of lipid. Lipids are a major class of biochemical compounds that includes oils, as well as fats. Among other things, organisms use lipids to store energy.
Lipid molecules consist mainly of repeating units called fatty acids. There are two types of fatty acids: saturated fatty acids and unsaturated fatty acids. Both types consist mainly of simple chains of carbon atoms bonded to one another and to hydrogen atoms. The two types of fatty acids differ in how many hydrogen atoms they contain and the number of bonds between carbon atoms.
3.5 Cultural Connection: Fats in Tanning

Ancient civilizations all over the world have used fats in the hide tanning process. If raw hides (animal skins) aren’t tanned, they get very brittle and can breakdown. Tanning results in a hide that is soft, flexible, and resists decay.
One method of tanning is called “brain tanning”. It’s name is quite self-explanatory — a mixture of boiled animal brains is used to tan a hide. A type of fat in the brain, called lecithin, is a natural tanning agent. Once the hide has been rubbed with the brain mixture, it is smoked and then it is ready for use!
Brain tanning is preferred in many cultures because it creates hides which are waterproof and it doesn’t create environmentally harmful byproducts.
Saturated Fatty Acids
In saturated fatty acids, carbon atoms are bonded to as many hydrogen atoms as possible. All the carbon-to-carbon atoms share just single bonds between them. This causes the molecules to form straight chains, as shown in the figure below. The straight chains can be packed together very tightly, allowing them to store energy in a compact form. Saturated fatty acids have relatively high melting points, which explains why they are solids at room temperature. Animals use saturated fatty acids to store energy. Some dietary examples of saturated fats include butter and lard.
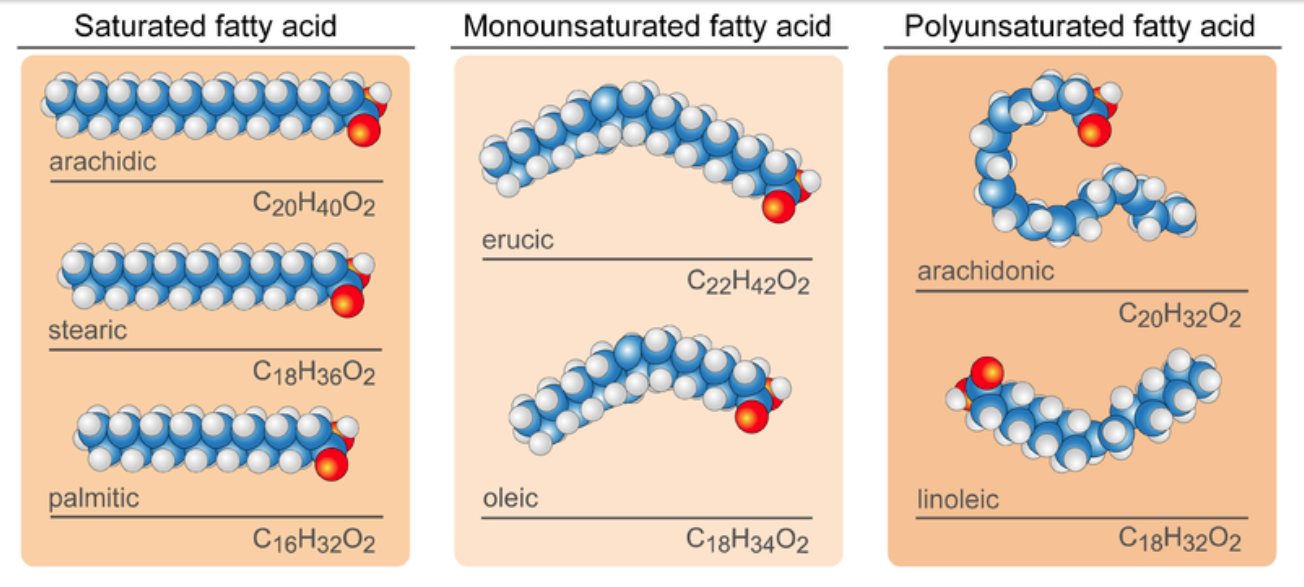
Unsaturated Fatty Acids
In unsaturated fatty acids, some carbon atoms are not bonded to as many hydrogen atoms as possible. Instead, they form double or even triple bonds with other carbon atoms. This causes the chains to bend (see Figure 3.5.3). The bent chains cannot be packed together very tightly. Unsaturated fatty acids have relatively low melting points, which explains why they are liquids at room temperature. Plants use unsaturated fatty acids to store energy.
Monounsaturated fatty acids contain one less hydrogen atom than the same-length saturated fatty acid chain. Monounsaturated fatty acids are liquids at room temperature, but start to solidify at refrigerator temperatures. Good food sources of monounsaturated fats include olive oils, peanut oils, and avocados.
Polyunsaturated fatty acids contain at least two fewer hydrogen atoms than the same-length saturated fatty acid chain. Polyunsaturated fatty acids are liquids at room temperature and remain in the liquid state in the refrigerator. Good food sources of polyunsaturated fats include safflower oils, soybean oils, and many nuts and seeds.
Types of Lipids
Lipids may consist of fatty acids alone, or they may contain other chemical components, as well. For example, some lipids contain alcohol or phosphate groups. Types of lipids include triglycerides, phospholipids, and steroids. Each type has different functions in living things.
Triglycerides
Triglycerides are formed by combining a molecule of glycerol with three fatty acid molecules, as shown below. Glycerol (also called glycerine) is a simple compound known as a sugar alcohol. It is a colourless, odorless liquid that is sweet tasting and nontoxic. Triglycerides are the main constituent of body fat in humans and other animals. They are also found in fats derived from plants. There are many different types of triglycerides, with the main division being between those that contain saturated fatty acids and those that contain unsaturated fatty acids.
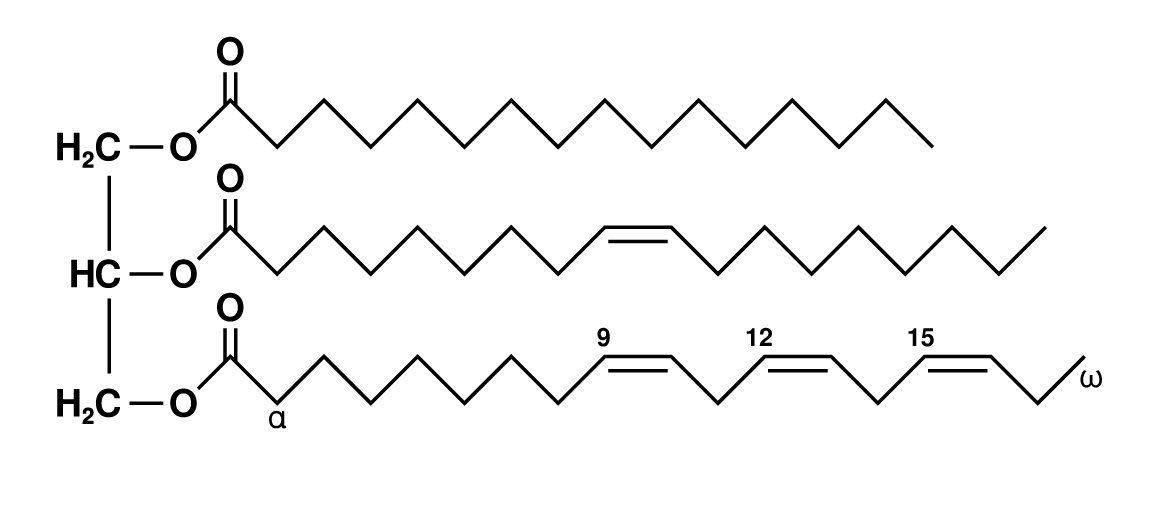
In the human bloodstream, triglycerides play an important role in metabolism as energy sources and transporters of dietary fat. They contain more than twice as much energy as carbohydrates, the other major source of energy in the diet. When you eat, your body converts any calories it doesn’t need to use right away into triglycerides, which are stored in your fat cells. When you need energy between meals, hormones trigger the release of some of these stored triglycerides back into the bloodstream.
Phospholipids
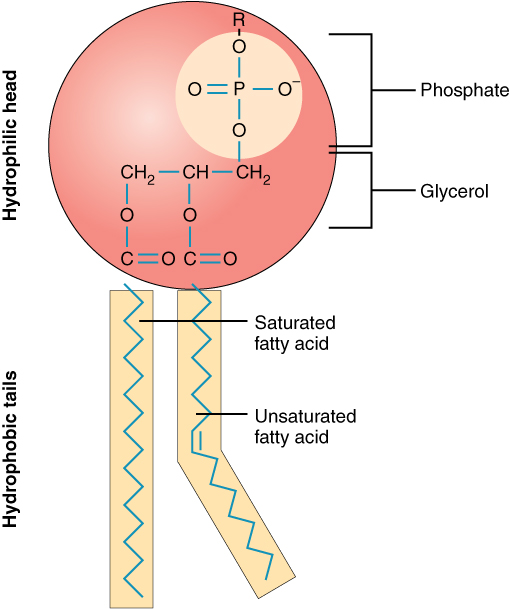
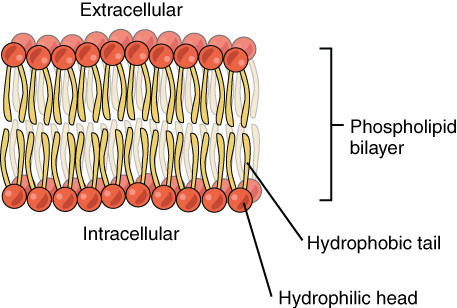
Phospholipids are a major component of the cell membranes of all living things. Each phospholipid molecule has a “tail” consisting of two long fatty acids, and a “head” consisting of a phosphate group and glycerol molecule (see Figure 3.5.5). The phosphate group is a small, negatively-charged molecule causing it to be hydrophilic, or attracted to water. The fatty acid tail of the phospholipid is hydrophobic, or repelled by water. These properties allow phospholipids to form a two-layered cell membrane, which is also called a bilayer.
As shown in Figure 3.5.6, a phospholipid bilayer forms when many phospholipid molecules line up tail to tail, forming an inner and outer surface of hydrophilic heads. The hydrophyilic heads point toward both the watery extracellular space and the watery inside space (lumen) of the cell. The hydrophobic fatty acids are nestled in the inner space of the bilayer.
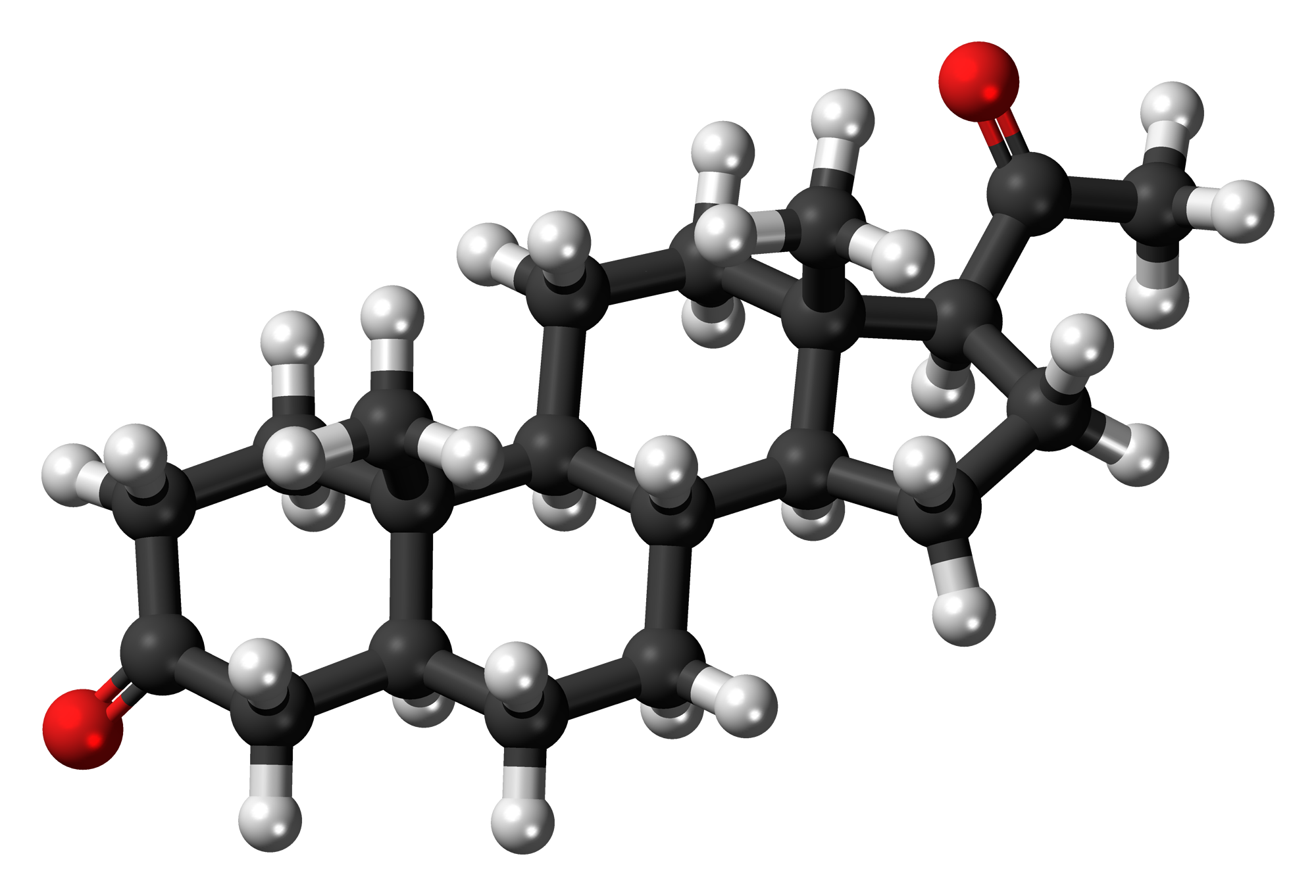
Steroids
Steroids are lipids with a ring structure. Each steroid has a core of 17 carbon atoms, which are arranged in four rings of five or six carbons each (pictured in Figure 3.5.7). Steroids vary by the other components attached to this four-ring core. Hundreds of steroids are found in plants, animals, and fungi, but most steroids have one of just two principal biological functions. Some steroids (such as cholesterol) are important components of cell membranes, while many other steroids are hormones, which are messenger molecules. In humans, steroid hormones include cortisone — a fight-or-flight hormone — and the sex hormones estrogen, progesterone and testosterone.
Feature: My Human Body
During a routine checkup with your family doctor, your blood was collected for a lipid profile. The results are back, and your triglyceride level is 180 mg/dL. Your doctor says this is a little high. A blood triglyceride level of 150 mg/dL or lower is considered normal. Higher levels of triglycerides in the blood have been linked to an increased risk of atherosclerosis, heart disease, and stroke.

If a blood test reveals that you have high triglycerides, the levels can be lowered through healthy lifestyle choices and/or prescription medications. Healthy lifestyle choices to control triglyceride levels include:
- Weight loss: If you are overweight, losing even 5 or 10 pounds (about 2.2 to 4.5 kg) may help lower your triglyceride level.
- Calorie reduction: Extra calories are converted to triglycerides and stored as fat, so reducing your calories should also reduce your triglyceride level.
- Reduction in sugars and refined foods: Simple carbohydrates, such as sugars and foods made with white flour, can increase triglyceride levels.
- Healthier fats: Trade saturated fats found in animal foods for healthier unsaturated fats found in plants and oily fish. For example, substitute olive oil for butter and salmon for red meat.
- Cut back on alcohol: Alcohol is high in calories and sugar. It has a strong effect on triglyceride levels.
- Regular exercise: Aim for at least 30 minutes of physical activity on most or all days of the week.
If healthy lifestyle changes aren’t enough to bring down high triglyceride levels, drugs prescribed by your doctor are likely to help.
3.5 Summary
- Lipids are a major class of biochemical compounds that includes oils and fats. Organisms use lipids for storing energy and for making cell membranes and hormones, which are chemical messengers.
- Lipid molecules consist mainly of repeating units called fatty acids. Depending on the proportion of hydrogen atoms they contain, fatty acids may be saturated or unsaturated. Animals store fat as saturated fatty acids, while plants store fat as unsaturated fatty acids.
- Types of lipids include triglycerides, phospholipids, and steroids. Each type consists of fatty acids and certain other molecules. Each also has different functions.
- Triglycerides contain glycerol (an alcohol), in addition to fatty acids. Humans and other animals store fat as triglycerides in fat cells.
- In addition to fatty acids, phospholipids contain phosphate and glycerol. They are the main component of cell membranes in all living things.
- Steroids are lipids with a four-ring structure. Some steroids (such as cholesterol) are important components of cell membranes. Many other steroids are hormones. An example of a human hormone is cortisone, which is the fight-or-flight hormone.
3.5 Review Questions
- What are lipids?
-
- Compare and contrast saturated and unsaturated fatty acids.
- Identify three major types of lipids. Describe differences in their structures.
- How do triglycerides play an important role in human metabolism?
- Explain how phospholipids form cell membranes.
- What is cholesterol? What is its major function?
- Give three examples of steroid hormones in humans.
- Which type of fatty acid do you think is predominant in the cheeseburger and fries shown above? Explain your answer.
- Which type of fat would be the most likely to stay liquid in colder temperatures: bacon fat, olive oil, or soybean oil? Explain your answer.
- Why do you think that the shape of the different types of fatty acid molecules affects how easily they solidify? Can you think of an analogy for this?
- High cholesterol levels in the bloodstream can cause negative health effects. Explain why we wouldn’t want to get rid of all of the cholesterol in our bodies.
3.5 Explore More
Cortisone and Healing – An overview of the science, by Sportology and OrthoCarolina, 2015
What is fat? – George Zaidan, TED-Ed, 2013
Attributions
Figure 3.5.1
cheeseburger by kayleigh harrington on Unsplash is used under the Unsplash License (https://unsplash.com/license).
Figure 3.5.2
Buffalo_Hide_Beaded_Guncase by Unknown onWikimedia Commons, is used under the Missouri History Museum‘s MHS Open Access Policy. Image is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 3.5.3
Fatty acids by CK-12 Foundation is used under a CC BY-NC 3.0 (https://creativecommons.org/licenses/by-nc/3.0/) license.
Figure 3.5.4
Fat_triglyceride_shorthand_formula by Wolfgang Schaefer on Wikimedia Commons, is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
Figure 3.5.5
Phospholipid_Structure by OpenStax on Wikimedia Commons, is used under a CC BY 4.0 (https://creativecommons.org/licenses/by/4.0) license.
Figure 3.5.6
Phospholipid_Bilayer by OpenStax on Wikimedia Commons, is used under a CC BY 4.0 (https://creativecommons.org/licenses/by/4.0) license.
Figure 3.5.7
Progesterone, 5alpha-Dihydroprogesterone 3D ball by Jynto is used under a CC0 1.0 Universal Public Domain Dedication license (https://creativecommons.org/publicdomain/zero/1.0/deed.en).
Figure 3.5.8
Healthy plate by Edgar Castrejon on Unsplash is used under the Unsplash License (https://unsplash.com/license).
References
Betts, J.G., Young, K.A., Wise, J.A., Johnson, E., Poe, B., Kruse, D.H., Korol, O., Johnson, J.E., Womble, M., DeSaix, P. (2013, April 25). Figure 3.2. Phospholipid structure [digital image]. In Anatomy and Physiology. OpenStax. https://openstax.org/books/anatomy-and-physiology/pages/3-1-the-cell-membrane
Betts, J.G., Young, K.A., Wise, J.A., Johnson, E., Poe, B., Kruse, D.H., Korol, O., Johnson, J.E., Womble, M., DeSaix, P. (2013, April 25). Figure 3.3. Phospolipid bilayer [digital image]. In Anatomy and Physiology. OpenStax. https://openstax.org/books/anatomy-and-physiology/pages/3-1-the-cell-membrane
Mayo Clinic. (n.d.). Arteriosclerosis / atherosclerosis [online article]. https://www.mayoclinic.org/diseases-conditions/arteriosclerosis-atherosclerosis/symptoms-causes/syc-20350569
Mayo Clinic. (n.d.). Heart disease [online article]. https://www.mayoclinic.org/diseases-conditions/heart-disease/symptoms-causes/syc-20353118
Mayo Clinic. (n.d.). Stroke [online article]. https://www.mayoclinic.org/diseases-conditions/stroke/symptoms-causes/syc-20350113
Sportology/OrthoCarolina. (2015, February 26). Cortisone and healing – An overview of the science. YouTube. https://www.youtube.com/watch?v=zqSoyaDu4b0&feature=youtu.be
TED-Ed. (2013, May 22). What is fat? – George Zaidan. YouTube. https://www.youtube.com/watch?v=QhUrc4BnPgg&feature=youtu.be
A substance that is insoluble in water. Examples include fats, oils and cholesterol. Lipids are made from monomers such as glycerol and fatty acids.
Long chains of hydrocarbons with a carboxyl group and a methyl group at opposite ends. Can be either saturated, containing mostly single bonds between adjacent carbons, or unsaturated, containing many double bonds between adjacent carbons.
A type of fat in which the fatty acid chains have all or predominantly single bonds.
The ability to do work.
A fat or fatty acid in which there is at least one double bond within the fatty acid chain. A fatty acid chain is monounsaturated if it contains one double bond, and polyunsaturated if it contains more than one double bond.
A type of lipid consisting of a glycerol and three fatty acids. Triglycerides are a form of energy storage used in animals (fats) and plants (oils).
A biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1. Complex carbohydrates are polymers made from monomers of simple carbohydrates, also termed monosaccharides.
A type of biological molecule consisting of two hydrophobic fatty acid "tails" and a hydrophilic "head" consisting of a phosphate group. Phospholipids are a major component of all cell membranes.
Attracted to water.
Repelled by water.
A biologically active organic compound with four rings arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes which alter membrane fluidity; and as signaling molecules.
A hormone is a signaling molecule produced by glands in multicellular organisms that target distant organs to regulate physiology and behavior.


