3.12 Acids and Bases

Created by: CK-12/Adapted by Christine Miller
Danger! Acid!
You probably know that batteries contain dangerous chemicals, including strong acids. Strong acids can hurt you if they come into contact with your skin or eyes. Therefore, it may surprise you to learn that your life depends on acids. There are many acids inside your body, and some of them are as strong as battery acid. Acids are needed for digestion and some forms of energy production. Genes are made of nucleic acids, proteins of amino acids, and lipids of fatty acids.
Water and Solutions
Acids (such as battery acid) are solutions. A solution is a mixture of two or more substances that has the same composition throughout. Many solutions are a mixture of water and some other substance. Not all solutions are acids. Some are bases and some are neither acids nor bases. To understand acids and bases, you need to know more about pure water.
In pure water (such as distilled water), a tiny fraction of water molecules naturally breaks down to form ions. An ion is an electrically charged atom or molecule. The breakdown of water is represented by the chemical equation:
2 H2O → H3O+ + OH–
The products of this reaction are a hydronium ion (H3O+) and a hydroxide ion (OH–). The hydroxide ion, which has a negative charge, forms when a water molecule gives up a positively charged hydrogen ion (H+). The hydronium ion, which has a positive charge, forms when another water molecule accepts the hydrogen ion.
Acidity and pH
The concentration of hydronium ions in a solution is known as acidity. In pure water, the concentration of hydronium ions is very low; only about one in ten million water molecules naturally breaks down to form a hydronium ion. As a result, pure water is essentially neutral. Acidity is measured on a scale called pH, as shown in Figure 3.12.2. Pure water has a pH of 7, so the point of neutrality on the pH scale is 7.
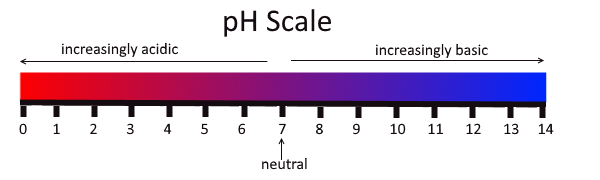
This pH scale shows the acidity of many common substances. The lower the pH value, the more acidic a substance is.

Acids
If a solution has a higher concentration of hydronium ions than pure water, it has a pH lower than 7. A solution with a pH lower than 7 is called an acid. As the hydronium ion concentration increases, the pH value decreases. Therefore, the more acidic a solution is, the lower its pH value is.
Did you ever taste vinegar? Like other acids, it tastes sour. Stronger acids can be harmful to organisms. Even stomach acid would eat through the stomach if it were not lined with a layer of mucus. Strong acids can also damage materials, even hard materials such as glass.
Bases
If a solution has a lower concentration of hydronium ions than pure water, it has a pH higher than 7. A solution with a pH higher than 7 is called a base. Bases, such as baking soda, have a bitter taste. Like strong acids, strong bases can harm organisms and damage materials. For example, lye can burn the skin, and bleach can remove the colour from clothing.
Buffers
A buffer is a solution that can resist changes in pH. Buffers are able to maintain a certain pH by absorbing any H+ or OH- ions added to the solution. Buffers are extremely important in biological systems in order to maintain a pH conducive to life. Bicarbonate is an example of a buffer which is used to maintain pH of the blood. In this buffering system, if blood becomes too acidic, carbonic acid will convert to carbon dioxide and water. If the blood becomes too basic, carbonic acid will convert to bicarbonate and H+ ions:
CO2 + H2O ↔ H2CO3 ↔ HCO3– + H+
Acids, Bases, and Enzymes
Many acids and bases in living things provide the pH that enzymes need. Enzymes are biological catalysts that must work effectively for biochemical reactions to occur. Most enzymes can do their job only at a certain level of acidity. Cells secrete acids and bases to maintain the proper pH for enzymes to do their work.
Every time you digest food, acids and bases are at work in your digestive system. Consider the enzyme pepsin, which helps break down proteins in the stomach. Pepsin needs an acidic environment to do its job. The stomach secretes a strong acid called hydrochloric acid that allows pepsin to work. When stomach contents enter the small intestine, the acid must be neutralized, because enzymes in the small intestine need a basic environment in order to work. An organ called the pancreas secretes a base named bicarbonate into the small intestine, and this base neutralizes the acid.
Feature: My Human Body
Do you ever have heartburn? The answer is probably “yes.” More than 60 million Americans have heartburn at least once a month, and more than 15 million suffer from it on a daily basis. Knowing more about heartburn may help you prevent it or know when it’s time to seek medical treatment.
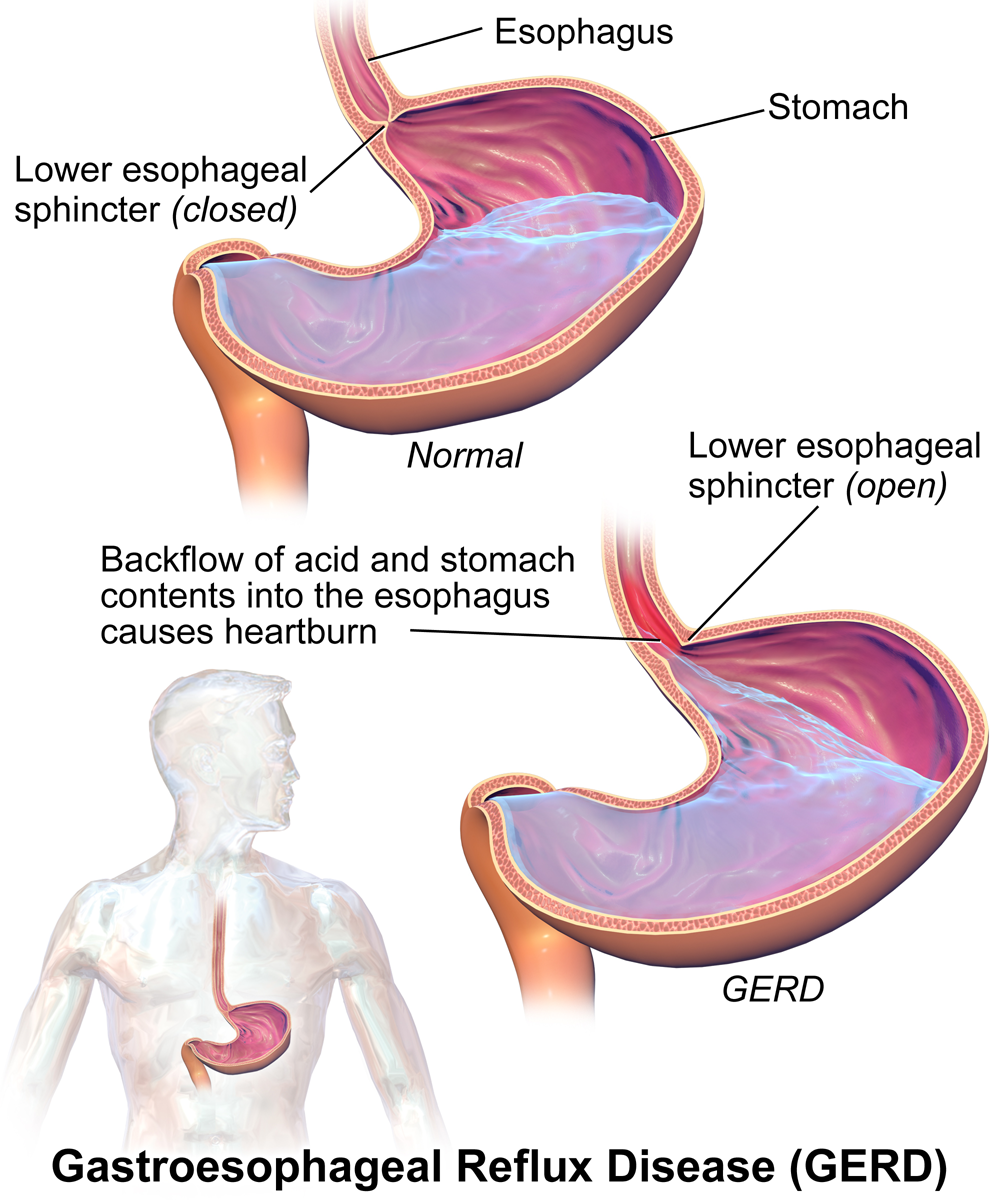
Heartburn doesn’t have anything to do with the heart, but it does cause a burning sensation in the vicinity of the chest. Normally, the acid secreted into the stomach remains in the stomach where it is needed to allow pepsin to do its job of digesting proteins. A long tube called the esophagus carries food from the mouth to the stomach. A sphincter, or valve, between the esophagus and stomach opens to allow swallowed food to enter the stomach and then closes to prevent stomach contents from backflowing into the esophagus. If this sphincter is weak or relaxes inappropriately, stomach contents flow into the esophagus. Because stomach contents are usually acidic, this causes the burning sensation known as heartburn. People who are prone to heartburn and suffer from it often may be diagnosed with GERD, which stands for gastroesophageal reflux disease.
GERD — as well as occasional heartburn — often can be improved by dietary and other lifestyle changes that decrease the amount and acidity of reflux from the stomach into the esophagus.
- Some foods and beverages seem to contribute to GERD, so these should be avoided. Problematic foods include chocolate, fatty foods, peppermint, coffee, and alcoholic beverages.
- Decreasing portion size and eating the last meal of the day at least a couple of hours before bedtime may reduce the risk of reflux occurring.
- Smoking tends to weaken the lower esophageal sphincter, so quitting the habit may help control reflux.
- GERD is often associated with being overweight. Losing weight often brings improvement.
- Some people are helped by sleeping with the head of the bed elevated. This allows gravity to help control the backflow of acids into the esophagus from the stomach.
If you have frequent heartburn and lifestyle changes don’t help, you may need medication to control the condition. Over-the-counter (OTC) antacids may be all that you need to control the occasional heartburn attack. OTC medications are usually bases that neutralize stomach acids. They may also create bubbles that help block stomach contents from entering the esophagus. For some people, OTC medications are not enough, and prescription medications are instead required for the control of GERD. These prescription medications generally work by inhibiting acid secretion in the stomach.
Be sure to see a doctor if you can’t control your heartburn, or you have it often. Untreated GERD not only interferes with quality of life, it may also lead to more serious complications, ranging from esophageal bleeding to esophageal cancer.
3.12 Summary
- A solution is a mixture of two or more substances that has the same composition throughout. Many solutions consist of water and one or more dissolved substances.
- Acidity is a measure of the hydronium ion concentration in a solution. Pure water has a very low concentration and a pH of 7, which is the point of neutrality on the pH scale.
- Acids have a higher hydronium ion concentration than pure water and a pH lower than 7. Bases have a lower hydronium ion concentration than pure water and a pH higher than 7.
- Many acids and bases in living things are secreted to provide the proper pH for enzymes to work properly. Enzymes are the biological catalysts (like pepsin) needed to digest protein in the stomach. Pepsin requires an acidic environment.
3.12 Review Questions
-
- What is a solution?
- Define acidity.
- Explain how acidity is measured.
- Compare and contrast acids and bases.
- Hydrochloric acid is secreted by the stomach to provide an acidic environment for the enzyme pepsin. What is the pH of this acid? How strong of an acid is it compared with other acids?
- Define an ion. Identify the ions in the equation below, and explain what makes them ions:
- 2 H2O → H3O+ + OH–
- Explain why the pancreas secretes bicarbonate into the small intestine.
- Do you think pepsin would work in the small intestine? Why or why not?
- You may have mixed vinegar and baking soda and noticed that they bubble and react with each other. Explain why this happens. Explain also what happens to the pH of this solution after you mix the vinegar and baking soda.
- Pregnancy hormones can cause the lower esophageal sphincter to relax. What effect do you think this has on pregnant women? Explain your answer.
3.12 Explore More
pH and Buffers by Bozeman Science, 2014.
The strengths and weaknesses of acids and bases – George Zaidan and Charles Morton, TED-Ed, 2013.
Attributions
Figure 3.12.1
Leaky battery by Carbon Arc on Flickr is used under a CC BY-NC-SA 2.0 (https://creativecommons.org/licenses/by-nc-sa/2.0/) license.
Figure 3.12.2
PH_Scale by Christinelmiller on Wikimedia Commons is used under a © CC0 1.0 (https://creativecommons.org/publicdomain/zero/1.0/) public domain dedication license.
Figure 3.12.3
Ph scale with examples by OpenStax College, on Wikimedia Commons, is used under a CC BY 3.0 (https://creativecommons.org/licenses/by/3.0) license.
Figure 3.12.4
GERD by BruceBlaus on Wikimedia Commons is used under a CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0) license.
References
Betts, J.G., Young, K.A., Wise, J.A., Johnson, E., Poe, B., Kruse, D.H., Korol, O., Johnson, J.E., Womble, M., DeSaix, P. (2013, April 25). Figure 26.15 The pH Scale [digital image]. In Anatomy and Physiology. OpenStax. https://openstax.org/books/anatomy-and-physiology/pages/26-4-acid-base-balance
Bozeman Science. (2014, February 22). pH and buffers. YouTube. https://www.youtube.com/watch?v=rIvEvwViJGk&feature=youtu.be
TED-Ed. (2013, October 24). The strengths and weaknesses of acids and bases – George Zaidan and Charles Morton. YouTube. https://www.youtube.com/watch?v=DupXDD87oHc&feature=youtu.be
An acid is a chemical substance which contains hydrogen and can react with other substances to form salts. Some acids burn or dissolve other substances that they come into contact with.
The ability to do work.
A complex organic substance present in living cells, especially DNA or RNA, whose molecules consist of many nucleotides linked in a long chain.
Amino acids are organic compounds that combine to form proteins.
Long chains of hydrocarbons with a carboxyl group and a methyl group at opposite ends. Can be either saturated, containing mostly single bonds between adjacent carbons, or unsaturated, containing many double bonds between adjacent carbons.
A mixture of two or more substances that has the same composition throughout.
the common name for the aqueous cation H3O+.
The level of acid in a substance.
A scale used to specify how acidic or basic a water-based solution is. Acidic solutions have a lower pH, while basic solutions have a higher pH.
Having a higher proportion of hydronium ions than hydroxide ions; having the properties of an acid; having a pH below 7.
Substances that, in aqueous solution, release hydroxide ions, are slippery to the touch, can taste bitter if an alkali.
A solution which can resist changes in pH.
Biological molecules that lower amount the energy required for a reaction to occur.
The transformation of one molecule to a different molecule inside a cell.
The smallest unit of life, consisting of at least a membrane, cytoplasm, and genetic material.
A class of biological molecule consisting of linked monomers of amino acids and which are the most versatile macromolecules in living systems and serve crucial functions in essentially all biological processes.

