3.9 Energy in Chemical Reactions
Created by: CK-12/Adapted by Christine Miller
Slow Burn

This old truck gives off a small amount of heat as it rusts. The rusting of iron is a chemical process. It occurs when iron and oxygen go through a chemical reaction similar to burning, or combustion. Obviously, the chemical reaction that occurs when something burns gives off energy. You can feel the heat, and you may be able to see the light of flames. The rusting of iron is a much slower process, but it still gives off energy. It’s just that it releases energy so slowly that you can’t detect a change in temperature.
The Role of Energy in Chemical Reactions
Matter rusting or burning are common examples of chemical changes. Chemical changes involve chemical reactions, in which some substances, called reactants, change at the molecular level to form new substances, which are called products. All chemical reactions involve energy, but not all chemical reactions release energy, like rusting and burning. In some chemical reactions, energy is absorbed rather than released.
Exothermic Reactions

A chemical reaction that releases energy is called an exothermic reaction. This type of reaction can be represented with this general chemical equation:
Reactants → Products + Heat
Another example of an exothermic reaction is chlorine combining with sodium to form table salt. The decomposition of organic matter also releases energy because of exothermic reactions. Sometimes on a chilly morning, you can see steam rising from a compost pile because of these chemical reactions (see photo in Figure 3.9.2).
This compost pile is steaming because it is much warmer than the chilly air around it. The heat comes from all the exothermic chemical reactions taking place inside the compost as it decomposes.
A special type of exothermic reaction is an exergonic reaction– not only do exergonic reactions release energy, but in addition, they occur spontaneously. Many cell processes rely on exergonic reactions: in a chemical process called cellular respiration, which is similar to combustion, the sugar glucose is “burned” to provide cells with energy.
Endothermic Reactions
A chemical reaction that absorbs energy is called an endothermic reaction. This type of reaction can also be represented by a general chemical equation:
Reactants + Energy → Products
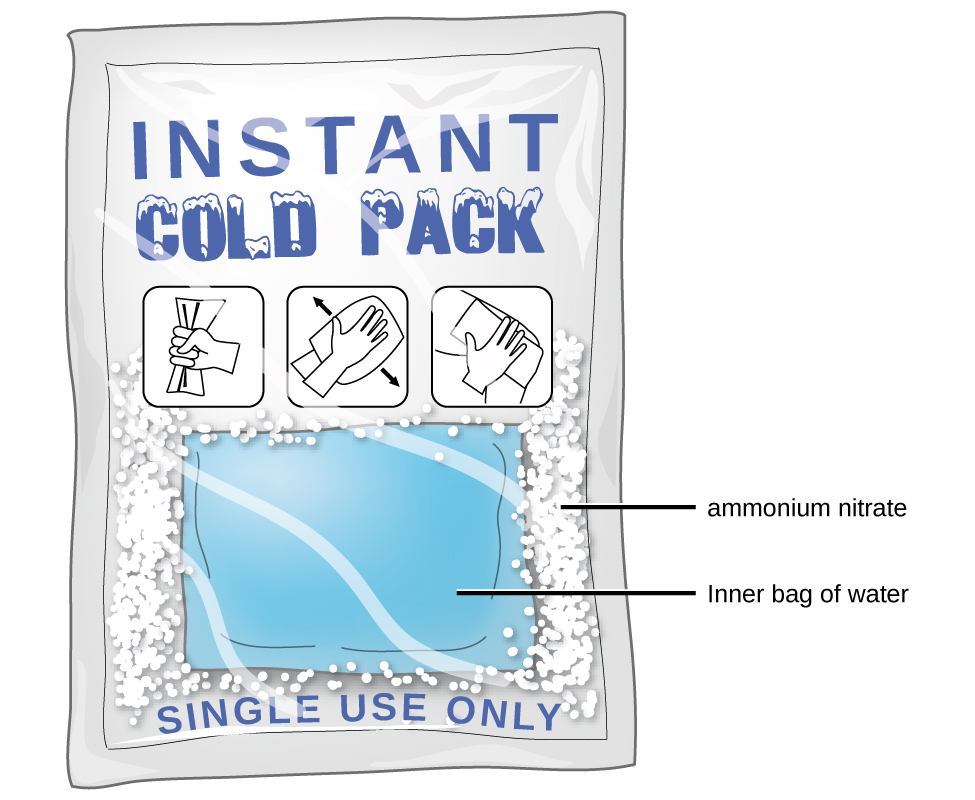
Did you ever use a chemical cold pack like the one pictured? The pack cools down because of an endothermic reaction. When a tube inside the pack is broken, it releases ammonium nitrate, a chemical that reacts with water inside the pack. This reaction absorbs heat energy and quickly cools down the contents of the pack.
Many other chemical processes involve endothermic reactions. Most cooking and baking, for example, involves the use of energy to produce chemical reactions. You can’t bake a cake or cook an egg without adding heat energy.
Arguably, the most important endothermic reactions occur during photosynthesis. When plants produce sugar by photosynthesis, they take in light energy to power the necessary endothermic reactions. The sugar they produce provides plants and virtually all other living things with glucose for cellular respiration.
Activation Energy
All chemical reactions require energy to get started. Even reactions that release energy need a boost of energy in order to begin. The energy needed to start a chemical reaction is called activation energy. Activation energy is like the push a child needs to start going down a playground slide. The push gives the child enough energy to start moving, but once she starts, she keeps moving without being pushed again. Activation energy is illustrated in the graph in Figure 3.9.4.
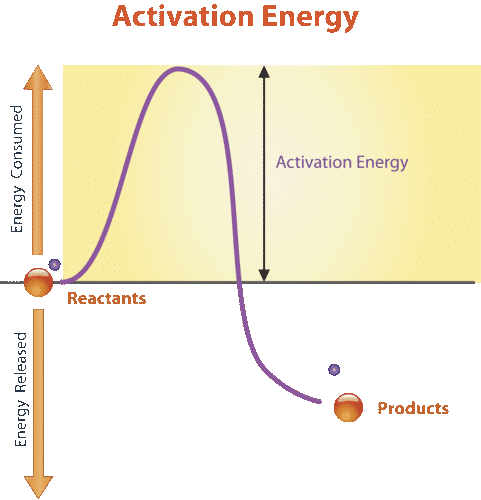
Why do chemical reactions need energy to get started? In order for reactions to begin, reactant molecules must bump into each other, so they must be moving — and movement requires energy. When reactant molecules bump together, they may repel each other because of intermolecular forces pushing them apart. Energy is also required to overcome these forces so the molecules can come together and react.
3.9 Summary
- All chemical reactions involve energy. Exothermic reactions release energy. Endothermic reactions absorb energy.
- All chemical reactions need activation energy to begin. Activation energy provides the “push” needed to get the reaction started.
3.9 Review Questions
- Compare endothermic and exothermic chemical reactions. Give an example of a process that involves each type of reaction.
- Define activation energy.
- Explain why chemical reactions require activation energy.
- Heat is a form of ____________ .
- In which type of reaction is heat added to the reactants?
- In which type of reaction is heat produced?
- If there was no energy added to an endothermic reaction, would that reaction occur? Why or why not?
- If there was no energy added to an exothermic reaction, would that reaction occur? Why or why not?
- Explain why a chemical cold pack feels cold when activated.
- Explain why cellular respiration and photosynthesis are “opposites” of each other.
- Explain how the sun gives our cells energy indirectly.
3.9 Explore More
Activation energy: Kickstarting chemical reactions – Vance Kite, TED-Ed, 2013.
The Sci Guys: Science at Home – SE1 – EP7: Hot Ice – Exothermic Reactions and Supercooled solutions, The Sci Guys, 2013
Attributions
Figure 3.9.1
Rusty truck by Ross Sokolovski on Unsplash is used under the Unsplash License (https://unsplash.com/license).
Figure 3.9.2
Compost/ Gently steaming compost! by John Winfield on Wikimedia Commons, is used under a
Figure 3.9.3
Cold Pack by OpenStax /CNX on Wikimedia Commons, is used under a CC BY 4.0 (https://creativecommons.org/licenses/by/4.0) license.
Figure 3.9.4
Activation energy by CK12 is used under a CC BY-NC 3.0 (https://creativecommons.org/licenses/by-nc/3.0/) license.
References
Brainard, J., Henderson, R. / CK12. (2018, August 22). Figure: Activation Energy [digital image]. In CK-12 College Human Biology. CK12. https://flexbooks.ck12.org/cbook/ck-12-college-human-biology-flexbook-2.0
OpenStax. (2019, Jul 30), Figure 6(b) A cold pack uses an endothermic process to create the sensation of cold. OpenStax Chemistry. OpenStax CNX. http://cnx.org/contents/85abf193-2bd2-4908-8563-90b8a7ac8df6@12.2. (Credit: a modification of work by “Skatebiker”/Wikimedia commons).
TED-Ed. (2013, January 9). Activation energy: Kickstarting chemical reactions – Vance Kite. YouTube. https://www.youtube.com/watch?v=D0ZyjpAin_Y&feature=youtu.be
The Sci Guys. (2013, April 4). The Sci Guys: Science at home – SE1 – EP7: Hot ice – Exothermic reactions and supercooled solutions. YouTube. https://www.youtube.com/watch?v=znsPa1BSaIM&feature=youtu.be
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another.
The ability to do work.
A chemical reaction that releases energy through light or heat.
A specific type of exothermic reaction which not only releases energy, but also occurs spontaneously.
Any reaction which requires or absorbs energy from its surroundings, usually in the form of heat.
The minimum energy required to cause a reaction to occur.

