3.2 Elements and Compounds
Created by: CK-12/Adapted by Christine Miller
What Are You Made of?

Your entire body is made of cells and cells are made of molecules.If you look at your hand, what do you see? Of course, you see skin, which consists of cells. But what are skin cells made of? Like all living cells, they are made of matter. In fact, all things are made of matter. Matter is anything that takes up space and has mass. Matter, in turn, is made up of chemical substances. A chemical substance is matter that has a definite composition that is consistent throughout. A chemical substance may be either an element or a compound.
Elements and Atoms
An element is a pure substance. It cannot be broken down into other types of substances. Each element is made up of just one type of atom.
Structure of an Atom
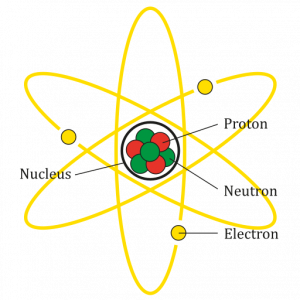
An atom is the smallest particle of an element that still has the properties of that element. Every substance is composed of atoms. Atoms are extremely small, typically about a ten-billionth of a metre in diametre. However, atoms do not have well-defined boundaries, as suggested by the atomic model shown below.
Every atom is composed of a central area — called the nucleus — and one or more subatomic particles called electrons, which move around the nucleus. The nucleus also consists of subatomic particles. It contains one or more protons and typically a similar number of neutrons. The number of protons in the nucleus determines the type of element an atom represents. An atom of hydrogen, for example, contains just one proton. Atoms of the same element may have different numbers of neutrons in the nucleus. Atoms of the same element with the same number of protons — but different numbers of neutrons — are called isotopes.
Protons have a positive electric charge and neutrons have no electric charge. Virtually all of an atom’s mass is in the protons and neutrons in the nucleus. Electrons surrounding the nucleus have almost no mass, as well as a negative electric charge. If the number of protons and electrons in an atom are equal, then an atom is electrically neutral, because the positive and negative charges cancel each other out. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively, and it is called an ion.
The negatively-charged electrons of an atom are attracted to the positively-charged protons in the nucleus by a force called electromagnetic force, for which opposite charges attract. Electromagnetic force between protons in the nucleus causes these subatomic particles to repel each other, because they have the same charge. However, the protons and neutrons in the nucleus are attracted to each other by a different force, called nuclear force, which is usually stronger than the electromagnetic force. Nuclear force repels the positively-charged protons from each other.
Periodic Table of the Elements
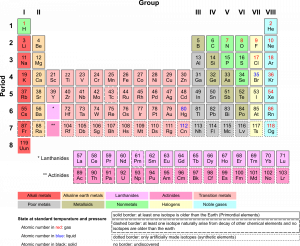
There are almost 120 known elements. As you can see in the Periodic Table of the Elements shown below, the majority of elements are metals. Examples of metals are iron (Fe) and copper (Cu). Metals are shiny and good conductors of electricity and heat. Nonmetal elements are far fewer in number. They include hydrogen (H) and oxygen (O). They lack the properties of metals.
The periodic table of the elements arranges elements in groups based on their properties. The element most important to life is carbon (C). Find carbon in the table. What type of element is it: metal or nonmetal?
Compounds and Molecules
A compound is a unique substance that consists of two or more elements combined in fixed proportions. This means that the composition of a compound is always the same. The smallest particle of most compounds in living things is called a molecule.
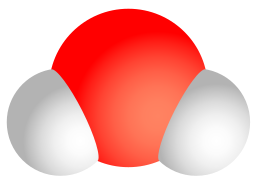
Consider water as an example. A molecule of water always contains one atom of oxygen and two atoms of hydrogen. The composition of water is expressed by the chemical formula H2O. A model of a water molecule is shown in Figure 3.2.4.
What causes the atoms of a water molecule to “stick” together? The answer is chemical bonds. A chemical bond is a force that holds together the atoms of molecules. Bonds in molecules involve the sharing of electrons among atoms. New chemical bonds form when substances react with one another. A chemical reaction is a process that changes some chemical substances into others. A chemical reaction is needed to form a compound, and another chemical reaction is needed to separate the substances in that compound.
3.2 Summary
- All matter consists of chemical substances. A chemical substance has a definite composition which is consistent throughout. A chemical substance may be either an element or a compound.
- An element is a pure substance that cannot be broken down into other types of substances.
- An atom is the smallest particle of an element that still has the properties of that element. Atoms, in turn, are composed of subatomic particles, including negative electrons, positive protons, and neutral neutrons. The number of protons in an atom determines the element it represents.
- Atoms have equal numbers of electrons and protons, so they have no charge. Ions are atoms that have lost or gained electrons, and as a result have either a positive or negative charge. Atoms with the same number of protons — but different numbers of neutrons — are called isotopes.
- There are almost 120 known elements. The majority of elements are metals. A smaller number are nonmetals. The latter include carbon, hydrogen, and oxygen.
- A compound is a substance that consists of two or more elements in a unique composition. The smallest particle of a compound is called a molecule. Chemical bonds hold together the atoms of molecules. Compounds can form only in chemical reactions, and they can break down only in other chemical reactions.
3.2 Review Questions
-
- What is an element? Give three examples.
- Define compound. Explain how compounds form.
- Compare and contrast atoms and molecules.
- The compound called water can be broken down into its constituent elements by applying an electric current to it. What ratio of elements is produced in this process?
- Relate ions and isotopes to elements and atoms.
- What is the most important element to life?
- Iron oxide is often known as rust — the reddish substance you might find on corroded metal. The chemical formula for this type of iron oxide is Fe2O3. Answer the following questions about iron oxide and briefly explain each answer.
- Is iron oxide an element or a compound?
- Would one particle of iron oxide be considered a molecule or an atom?
- Describe the relative proportion of atoms in iron oxide.
- What causes the Fe and O to stick together in iron oxide?
- Is iron oxide made of metal atoms, metalloid atoms, nonmetal atoms, or a combination of any of these?
- 14C is an isotope of carbon used in the radiocarbon dating of organic material. The most common isotope of carbon is 12C. Do you think 14C and 12C have different numbers of neutrons or protons? Explain your answer.
- Explain why ions have a positive or negative charge.
- Name the three subatomic particles described in this section.
3.2 Explore More
Just how small is an atom? TED-Ed, 2012
Attributions
Figure 3.2.1
Man Sitting, by Gregory Culmer, on Unsplash, is used under the Unsplash license (https://unsplash.com/license).
Figure 3.2.2
Lithium Atom diagram, by AG Caesar, is used under a CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0/deed.en)
Figure 3.2.3
Periodic Table Armtuk3, by Armtuk, is used under a CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0/) license.
Figure 3.2.4
Water molecule, by Sakurambo, is released into the public domain (https://en.wikipedia.org/wiki/Public_domain).
References
TED-Ed. (2012, April 16). Just how small is an atom. YouTube. https://www.youtube.com/watch?v=yQP4UJhNn0I&feature=youtu.be
The smallest unit of life, consisting of at least a membrane, cytoplasm, and genetic material.
Anything that takes up space and has mass.
A form of matter having constant chemical composition and characteristic properties which cannot be separated into its constituent elements without breaking chemical bonds.
Elements are chemically the simplest substances and hence cannot be broken down using chemical reactions. An element is a substance whose atoms all have the same number of protons.
The smallest particle of an element that still has the properties of that element.
A small dense region in the center of an atom containing protons and neutrons.
A sub-atomic particle with a charge of -1.
A sub-atomic particle with a charge of +1.
A sub-atomic particle with a charge of 0.
Variants of a type of atom which differ in the number of neutrons in the nucleus and therefore differ in atomic mass.
An atom or molecule with a net electric charge due to the loss or gain of one or more electrons.
A type of physical interaction that occurs between electrically charged particles.
A force that acts between the protons and neutrons of atoms.
A substance consisting of atoms or ions of two or more different elements in definite proportions joined by chemical bonds into a molecule.
A molecule is an electrically neutral group of two or more atoms held together by chemical bonds.
A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds.
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another.

